Guidelines have emerged to increase the sensitivity of assays for Clostridium difficile (C. difficile). The primary two guidelines in the U.S. support the use of testing for a common antigen known as glutamate dehyrogenase (GDH) as a primary screen.1,2 GDH has been shown to have sensitivity levels equivalent to molecular methods. It is produced by non-toxic strains, however, making the testing for the toxin also necessary.
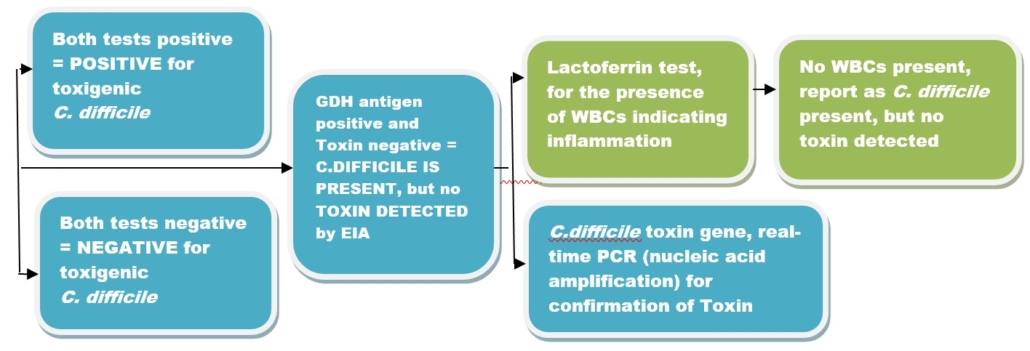
NICL Laboratories will be performing C. difficile testing using an algorithm. This algorithm will consist of a combination test which includes an Enzyme immunoassay test for the antigen GDH and C. difficile toxin (CDT). If both tests are negative, the patient will be considered negative for C. difficile Toxin A/B. If both tests are positive, the patient will be considered positive for C.difficile Toxin A/B. When the results disagree, this combination test will be followed, with a test for the toxin gene using a real-time polymerase chain (PCR) based qualitative test. The test targets C. difficile Toxin A gene (tcdA), and Toxin B gene (tcdB). This algorithm appears in teal blue in the chart below.
The laboratory will also be providing a Lactoferrin immunochromatographic assay for detecting the presence of WBCs in stool, to rule out inflammation. This additional testing, which appears in green in the chart below, will be performed only upon request.
The new test code for C. difficile, including the nucleic acid probe confirmation is 27449. This testing will continue to be available on a daily basis. The test code for Lactoferrin for detecting white blood cells in stool is 13630.
NOTE: This testing will only be performed on uniform (liquid or soft) stool specimen. Solid or hard formed samples will be rejected.
1 Cohen,et al. Clinical Practice Guidelines for Clostridium difficile infection in adults: 2010 update by the Society of Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infection Control and Hospital Epidemiology. 2010
2American Society for Microbiology. 20103Noren, Torbjorn, Alriksson, Ingegard, Andersson, Josefin, Akerlund, Thomas, Unemo, Magnus Rapid and Sensitive LoopMediated Isothermal Amp0lification Test for Clostridium difficile Detection Challenges Cytotoxin B Cell Test and Culture as Gold Standard, Journal of Clinical Microbiology, Feb 2011, p 710-711




