Respiratory Viral Pathogens Collection
NOTE: Wear a gown, gloves, mask (N95 preferred), and eyewear when collecting these specimen.
1. Obtain specimen using only the mini-tip swab provided in the kit. Due to supply shortages, please use one swab per patient, please!
- When collecting just a SARS CoV-2 sample, you may use an anterior nasal swab.
- All other viral pathogen tests require a thin-tipped nasopharyngeal swab.
| Swab Type | Collection Procedure |
|---|---|
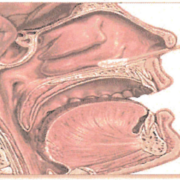
| Nasopharyneal (N/P) swab Has a very thin swab end; diameter of swab end is not much larger than swab handle | Push forward using gentle downward pressure to keep the swab on the floor of the nasal cavity until the tip reaches the posterior wall of the nasopharynx. Gently rub and roll the swab, and leave in place for several seconds to absorb fluid.* It is not necessary to swab both sides if the swab is fully saturated from one side, but if both sides are collected, use a single swab on both the left and right nasal cavity. A single swab may be used for SARS-CoV-2 and Influenza/RSV or Respiratory Viral Panel. |
| Anterior nasal swab Swab end resembles a Q-tip. Do not attempt to collect a nasopharyngeal specimen using this type of swab. | Insert the swab at least one-half inch inside the nostril (naris) and firmly sample the nasal membrane by rotating the swab and leaving in place for 10 to 15 seconds. Sample both nostrils with the same swab. This type of swab can only be used for SARS-CoV-2 testing. It cannot be used for Influenza, RSV or other Respiratory Viral Pathogen testing. |
2. Break the shaft at the molded mark so that it can be placed in the tube without restricting the cap, and replace the cap tightly to prevent leakage and specimen rejection.
3. Label the vial with patient information (Name, DOB, collection date & time, specimen source and the test requested). Refrigerate it immediately.
4. Store the specimen refrigerated, and send it to the lab as soon as possible.
5. Complete the requisition. Indicate the swab type, and if the patient is symptomatic or if testing is for preoperative procedures. Race and ethnicity are also required by Health and Human Services. This is essential!
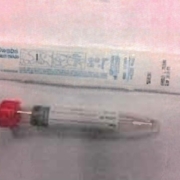
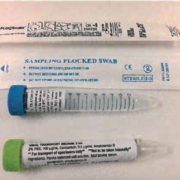
The above are M4RT media and other Viral transport media (check the label to see if the media should be kept refrigerated prior to collection). They are appropriate for Influenza, RSV, Respiratory Viral Panels and SARS-CoV-2 testing. NICL requires the use Viral transport media for its viral pathogens testing.